This is an Accord Healthcare Ltd website and is intended for Healthcare Professionals residing in the United Kingdom (UK). If you are a UK patient Click here
Prescribing Information is available in the header below. Adverse Event Reporting can be found at the bottom of the webpage.
- For the treatment of adult patients with advanced hormone-sensitive prostate cancer1
- For the treatment of high-risk localised and locally advanced hormone dependent prostate cancer in combination with radiotherapy1
- As neo-adjuvant treatment prior to radiotherapy in patients with high-risk localised or locally advanced hormone dependent prostate cancer1
Introducing AccordEngage, your go-to for:
- Medical education content
- Disease awareness
- Expert insight
Dosing
Convenient dosing that fits into patients daily routines.1
Starting Dose
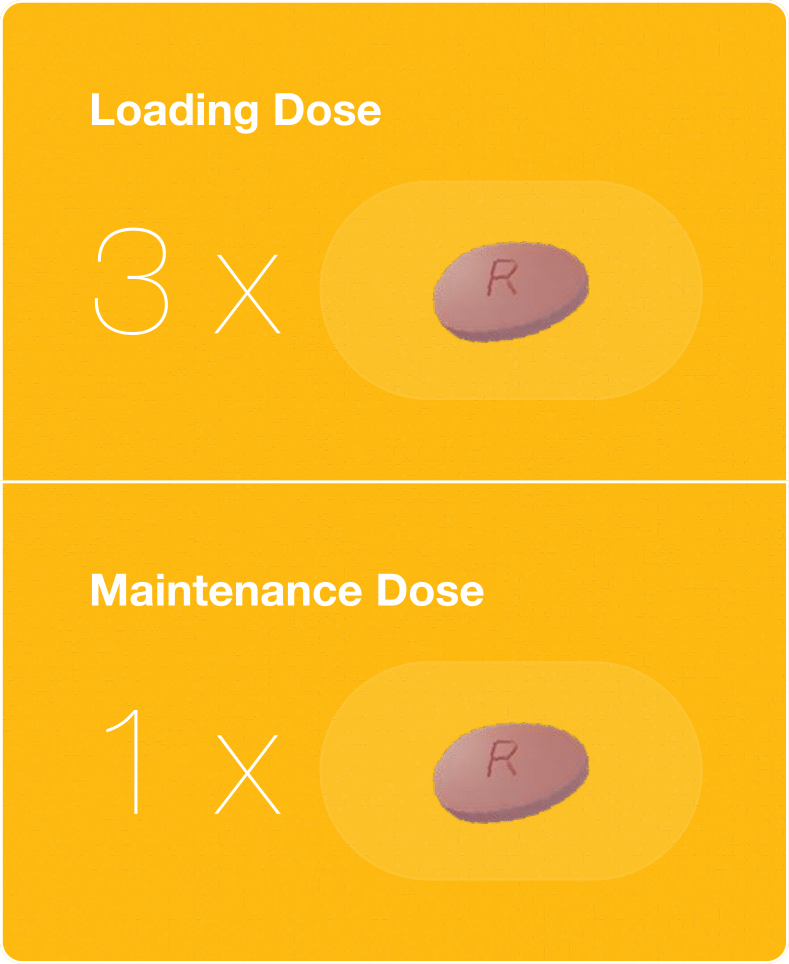
Initiate ORGOVYX® with a loading dose of 360 mg (three tablets) on the first day, followed thereafter by a 120 mg (one tablet) dose taken once daily at approximately the same time each day.1
Tablets shown are not to size

Starting Dose
Tablets shown are not to size

ORGOVYX® Is Administered Orally
Dose Adjustment
Missed Doses
If a dose is missed, ORGOVYX® must be taken as soon as the patient remembers. If the dose was missed by more than 12 hours, the missed dose must not be taken and the regular dosing schedule should be resumed the following day.1
If treatment with ORGOVYX® is interrupted for greater than 7 days, ORGOVYX® must be restarted with a loading dose of 360 mg on the first day, followed by a dose of 120 mg once daily.1
Please refer to the SmPC before prescribing
Efficacy
Primary endpoint:
Sustained testosterone suppression below castrate levels (<50 ng/dL) from Day 29 through 48 Weeks was achieved in 96.7% of patients receiving ORGOVYX® (95% CI, 94.9–97.9), as compared with 88.8% (95% CI, 84.6–91.8) of patients receiving leuprorelin.2
CV Risk
ORGOVYX® Was Associated With A Lower Incidence Of MACE Compared To Leuprorelin
ORGOVYX® Was Associated With A Lower Risk Of MACE Compared To Leuprorelin In A Post-Hoc Analysis
Discover resources and recommendations for assessing and managing CV risk in your patients with prostate cancer on AccordEngage
Safety Information
Contraindications
ORGOVYX® is contraindicated in patients with severe hypersensitivity to relugolix or any of the following excipients:1
Carnauba wax (E903)

Contraindications
Safety Information


Special Warnings and Precautions for Use
Prior to prescribing ORGOVYX®, patients should be assessed for CV and bone density risk factors.1
The benefit-risk ratio, including the potential for Torsade de points, should be assessed in patients with a history of risk factors for QT prolongation or in patients receiving concomitant medicinal products that may prolong the QT interval, prior to initiating ORGOVYX®.1
Caution is warranted in patients with severe renal impairment upon administration of a 120 mg dose of ORGOVYX® once daily.1
It is also recommended to monitor liver function in patients with known or suspected hepatic disorder and PSA in all patients where clinically appropriate.1
Adverse Reactions
The most commonly observed adverse reactions during ORGOVYX® therapy (≥10% incidence) include:1
Diarrhoea
Constipation
Musculoskeletal
pain
Please refer to the SmPC for a full list of adverse events associated with ORGOVYX® therapy.
Resources
AccordEngage is an online centralised platform for information about Accord medicines and related therapy areas, and features a wealth of resources dedicated to ORGOVYX®.
Frequently asked question
ORGOVYX® (relugolix) is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated in the United Kingdom:
- For the treatment of adult patients with advanced hormone-sensitive prostate cancer1
- For the treatment of high-risk localised and locally advanced hormone dependent prostate cancer in combination with radiotherapy1
- As neo-adjuvant treatment prior to radiotherapy in patients with high-risk localised or locally advanced hormone dependent prostate cancer1
ORGOVYX® contains the active ingredient relugolix. Each ORGOVYX® film-coated tablet contains 120 mg of relugolix.1
ORGOVYX® is administered orally. It’s important to advise patients that they should swallow their tablets whole and avoid crushing or chewing them. The tablet can be taken with or without food and with some liquid.2
Treatment should be initiated with a loading dose of 360 mg (three tablets) on the first day, followed thereafter by a 120 mg (one tablet) dose taken once daily at approximately the same time each day.1
In a prespecified safety analysis of the HERO study, after 48 weeks of treatment, the incidence of MACE was 2.9% (95% CI, 1.7–4.5) with ORGOVYX® and 6.2% (95% CI, 3.8–9.5) with leuprorelin.2
In the subgroup of patients with a reported medical history of MACE, the incidence of MACE during receipt of the trial drug was 3.6% (3 of 84 patients) with ORGOVYX® and 17.8% (8 of 45 patients) in the leuprorelin group, which indicates that the odds of having an event were 4.8x lower with ORGOVYX® than leuprorelin.2
Treatment with ORGOVYX® is not associated with the initial increases in FSH and LH concentrations and subsequent testosterone (“potential symptomatic flare”) observed upon initiation of treatment with an LHRH analogue.1
Therefore, adding an anti-androgen as surge protection is unnecessary when initiating ORGOVYX® therapy.1
The most commonly observed adverse reactions experienced during relugolix therapy are physiological effects of testosterone suppression, including hot flushes (54%), musculoskeletal pain (30%), and fatigue (26%). Other very common adverse reactions include diarrhoea and constipation (12% each).1
ORGOVYX® is contraindicated in patients with severe hypersensitivity to relugolix or any of the following excipients:1
- Mannitol (E421)
- Sodium starch glycolate (E468)
- Hydroxypropyl cellulose (E463)
- Magnesium stearate (E572)
- Hypromellose (E464)
- Titanium dioxide (E171)
- Iron oxide red (E172)
- Carnauba wax (E903)
Co-administration of ORGOVYX® and oral P-glycoprotein (P-gp) inhibitors should be avoided. If co-administration is unavoidable, take ORGOVYX® first with the oral P-gp inhibitor taken 6 hours thereafter. Treatment with ORGOVYX® may be interrupted for up to 2 weeks if a short course of treatment with a P-gp inhibitor is required.1
Co-administration of ORGOVYX® with combined P-gp and strong CYP3A inducers should be avoided. If co-administration is unavoidable, the dose of ORGOVYX® must be increased to 240 mg once daily. After discontinuation of the combined P-gp and strong CYP3A inducer, resume the recommended ORGOVYX® dose of 120 mg once daily.1
Please see the full SmPC for potential drug interactions.
ORGOVYX® does not require dose adjustment in elderly patients or patients with mild/moderate renal or hepatic impairment. However, caution is warranted in patients with severe renal impairment.1
Monitoring of liver function in patients with known or suspected hepatic disorder is advised during treatment. The pharmacokinetics of relugolix in patients with severe hepatic impairment has not been evaluated.1
The HERO study was a multinational, randomised, open-label, Phase 3 trial to evaluate the efficacy and safety of ORGOVYX®, compared to leuprorelin, in men with advanced prostate cancer.2
For further resources, including on-demand videos with insights from leading experts, guidance on best practice and practical resources, visit AccordEngage.co.uk.
References
- ORGOVYX® Summary of Product Characteristics.
- Shore ND, et al. N Engl J Med. 2020;382(4):2187–2196.
Acronyms
Adverse events should be reported.
Reporting forms and information can be found at yellowcard.mhra.gov.uk
Adverse events should also be reported to Accord-UK Ltd on 01271 385257 or email medinfo@accord-healthcare.com.
UK-05491 | August 2025
Accord-UK Ltd. Whiddon Valley, Barnstaple, Devon, EX32 8NS, United Kingdom
© Copyright Accord Healthcare Ltd 2025